Odontoglossum Ringspot Virus
Odontoglossum Ringspot Virus (ORSV) is described as one of the most common and important viruses infecting orchids worldwide. ORSV can be transmitted mechanically with infected sap and produces symptoms ranging from none to necrotic spotting with light green or yellow rings. Control measures are limited to sterilization of tools between plants and choosing only virus tested ORSV negative plants. Testing for ORSV should be done on all plants used for tissue culture propagation and any other plants of value.
Introduction
Odontoglossum ringspot virus (abbreviated ORSV) is one of the most common and important viruses infecting orchids worldwide. The other major viral pathogen of orchids is Cymbidium mosaic virus. ORSV was first described in 1951 as a viral pathogen of Odontoglossum grande orchid plants (Jensen, Gold 1951). ORSV in the past was also called the orchid strain of tobacco mosaic virus due to the similarity in virus particle shape and size. ORSV has rod-shaped particles that are 300 nanometers long and 18 nanometers wide.
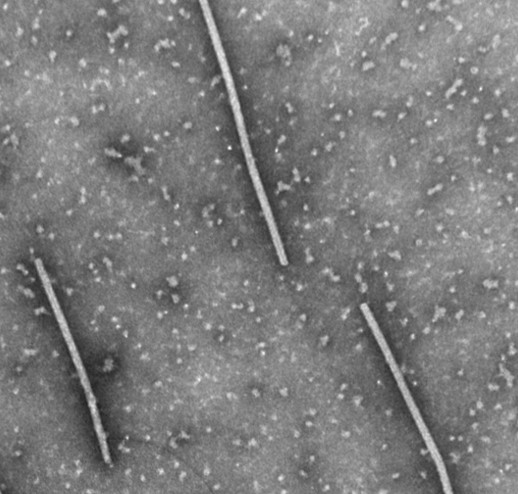 Negatively stained transmission electron microscopy of ORSV isolated from a Cymbidium hybrid orchid by Sara Bratsch.
Negatively stained transmission electron microscopy of ORSV isolated from a Cymbidium hybrid orchid by Sara Bratsch.
Symptoms
ORSV infecting Odontoglossum grande plants cause symptoms of small necrotic spots that appear on older leaves while younger leaves have light green or pale yellow rings. The rings continue to grow in size and can cover the entire leaf. Flowers may exhibit color breaking or necrotic spotting (Jensen, Gold 1951). Mild flower symptoms on both flower petals and sepals include irregular areas that are lighter or darker in color than normal. Severe flower color break will cause white variegation in both petals and sepals or brown necrotic streaking (McMillan, Vendrame 2005). Previous studies have shown no obvious foliar symptoms in cymbidium orchids mechanically inoculated with ORSV and followed for 4 years. The plants did have a reduction in growth along with several plants dying (Pearson, Cole 1991). The absence of obvious leaf symptoms in ORSV infected plants increases the likelihood of the virus infection going undetected.
 Symptoms of ORSV infection in several orchid species. A-Bollea violaceae showing very slight symptoms (which could also be due to lack of fertilizing). B-Epidendrum raniferum leaves showing extreme chlorosis and necrotic spotting. C-Cymbidium sp. with pin-prick chlorotic spots. D-Miltonia sp. showing necrotic spotting. E-Brassia verrucosa with chlorotic spots and leaf yellowing.
Symptoms of ORSV infection in several orchid species. A-Bollea violaceae showing very slight symptoms (which could also be due to lack of fertilizing). B-Epidendrum raniferum leaves showing extreme chlorosis and necrotic spotting. C-Cymbidium sp. with pin-prick chlorotic spots. D-Miltonia sp. showing necrotic spotting. E-Brassia verrucosa with chlorotic spots and leaf yellowing.
Control
ORSV can be transmitted mechanically with infected sap on tools. Seed or insect vector transmission of ORSV has not been extensively studied. At this time the major transmission method of ORSV is mechanical transmission or propagation by tissue culture of an infected mother plant.
Several studies have been done to determine the incidence of ORSV infection rate in several countries. A 1993 orchid survey by the University of Hawaii of 3,600 orchid plants from 3 collections, 22 commercial farms, and 6 nurseries identified a plant infection rate of 17% by ORSV and 15% infection by both ORSV and Cymbidium mosaic virus (CymMV) (CymMV was found in 45%)(Hu et al. 1993). A similar study by the Department of Botany in Singapore of 1146 orchid plants from 4 commercial cut flower farms identified a 4% ORSV infection rate and 14.2% infection rate for ORSV and CymMV (54.6% were infected with CymMV alone)(Wong et al. 1994). A more recent survey from Thailand of 280 Dendrobium orchids from cut flower farms in Thailand identified a 0% infection rate by ORSV (65.4% CymMV infection rate)(Khentry et al 2006). A study from Sikkim, India showed 42 of 100 orchids sampled were infected with ORSV with the majority of plants showing no symptoms (Sherpa et al. 2006). These results indicate that it is likely plants being sold are infected with ORSV but it is possible to keep the incidence to 0% through sterilization methods (as seen by the 0% infection rate from cut flower farms).
There has been some work done using tissue culture methods to eliminate ORSV from cultured plant material. These methods could be used to obtain virus free plantlets from an infected mother plant. ORSV is not easily eliminated through meristem tissue culture, however it is possible to obtain some virus free plantlets using tissue culture with chemotherapy (antiviral chemicals added to the media). ORSV has been shown to be present in the lateral and apical meristems of ORSV infected Cymbidium orchids making meristem culture alone an inefficient method of producing virus-free plantlets (Toussaint et al. 1984). Meristem tissue culture combined with antiviral chemicals (like ribavirin/virazole) have been successful in producing ORSV free Cymbidium plantlets, however the efficiency was dependent upon media and concentration of chemical used (Freitas-Astua et al. 1998). Further studies with Mokara orchids in tissue culture showed similar results with certain concentrations of ribavirin working more efficiently to eliminate ORSV from meristem or thin section cultures (Lim et al. 1993).
Testing methods for ORSV include indicator plants, reverse transcription polymerase chain reaction (RT-PCR), and antibody based methods including ELISA, immunosorbant transmission electron microscopy, and rapid antibody tests. The commercially available rapid antibody tests from Agdia for ORSV, which are available for orchid growers of all sizes, utilize a method similar to pregnancy tests with a band indicating a positive reaction from a sample of ground plant tissue. Plant Pathology diagnostic labs can also be consulted for orchid virus (and other pathogen) tests.
Control measures for ORSV include testing and isolating or destroying infected plants. Plants that will be used for mass propagation by tissue culture should be tested for viruses before they are used for propagation, even if they do not show symptoms, as not every plant will express symptoms. Strict sterilization methods are required for working with more than one orchid plant. Tools should be single use like individual razor blades which are disposed after using on one plant. Otherwise tools should be disinfected by dipping in a 1% NaOH (bleach) solution between plants to inactivate any virus present (Hu 1994). It is also important to sterilize pots, benches, and the working area to eliminate the risk of transmitting any virus present.
In the future it may be possible to buy genetically engineered orchids with resistance to ORSV and CymMV, however there are currently no resistant orchids being sold. The only way to protect your collection of orchids is to maintain sterilization techniques between plants and when purchasing new plants to buy only ones that have been virus tested. To determine if your plant has been virus tested you can look on the label or contact the seller directly to learn what viruses they are testing for. It is important to remember that although a plant may be expressing no symptoms, it may still contain a virus that can slowly weaken plants and be spread between plants.
For information on CymMV check out my posting about it here: https://plantpathlesstraveled.com/cymbidium-mosaic-virus/
All photos were taken by Sara Bratsch. For non commercial use only.
Please contact regarding all other uses.
Cite this article:
Bratsch, Sara. “Odontoglossum ringspot virus”. 12 June 2015. http://plantpathlesstraveled.com/odontoglossum-ringspot-virus/
Citations:
Freitas-Astua, J., & Rezende, J. A. M. (1998). Odontoglossum ringspot virus-free Cymbidium obtained through meristem tip chemotherapy. Fitopatologia Brasileira, 23(2), 158-160.
Hu, J. S., Ferreira, S., Xu, M. Q., Lu, M., Iha, M., Pflum, E., & Wang, M. (1994). Transmission, movement and inactivation of cymbidium mosaic and odontoglossum ringspot viruses. Plant disease, 78(6), 633-636.
Khentry, Y., Paradornuwat, A., Tantiwiwat, S., Phansiri, S., & Thaveechai, N. (2006). Incidence of Cymbidium mosaic virus and Odontoglossum ringspot virus in Dendrobium spp. in Thailand. Crop Protection, 25(9), 926-932.
Lim, S. T., Wong, S. M., & Goh, C. J. (1993). Elimination of cymbidium mosaic virus and odontoglossum ringspot virus from orchids by meristem culture and thin section culture with chemotherapy. Annals of applied biology, 122(2), 289-297.
McMillan Jr, R. T., & Vendrame, W. A. (2005). Color break in orchid flowers. In Proceedings of the Florida State Horticultural Society (Vol. 118, pp. 287-288).
Pearson, M. N., & Cole, J. S. (1991). Further observations on the effects of Cymbidium mosaic virus and Odontoglossum ringspot virus on the growth of Cymbidium orchids. Journal of Phytopathology, 131(3), 193-198.
Sherpa, A. R., Bag, T. K., Hallan, V., & Zaidi, A. A. (2006). Detection of Odontoglossum ringspot virus in orchids from Sikkim, India. Australasian Plant Pathology, 35(1), 69-71.
Toussaint, A., Dekegel, D., & Vanheule, G. (1984). Distribution of Odontoglossum ringspot virus in apical meristems of infected Cymbidium cultivars. Physiological plant pathology, 25(3), 297-305.
Wong, S. M., Chng, C. G., Lee, Y. H., Tan, K., & Zettler, F. W. (1994). Incidence of cymbidium mosaic and odontoglossum ringspot viruses and their significance in orchid cultivation in Singapore. Crop Protection, 13(3), 235-239.